
19 Depending on the post-annealing temperature, films of both, the tetragonal or monoclinic structure can be prepared.
14 and Meinel et al., 15–17 but taking advantage of sputter deposition in UHV, 18 we have recently demonstrated the reliable and reproducible growth of several-monolayer-thick films of bulk-like zirconia. 13 However, for investigating oxygen deficiency and related phenomena, thicker films are required. In the last years, many studies have focussed on ultrathin zirconia films, 8–10 which has helped to gain insights into molecular adsorption 11,12 and metal growth. Only then, surface science techniques such as scanning tunneling microscopy (STM), low-energy electron diffraction (LEED), or X-ray photoelectron spectroscopy (XPS) can be used. 6,7 For analysis methods involving charged particles, one has to rely on thin films to circumvent charging of the material.

The main difficulty of such studies is the lack of electronic conductivity: zirconia (pure or yttria-stabilized) has a band gap of 5–6 eV.
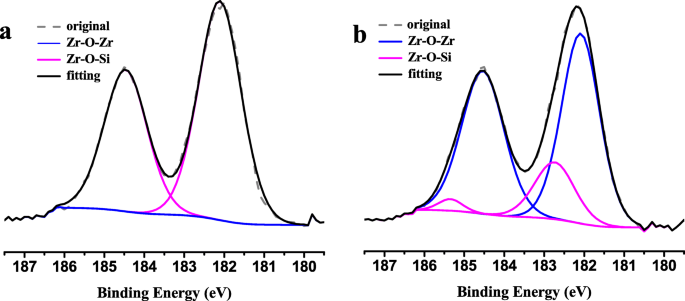
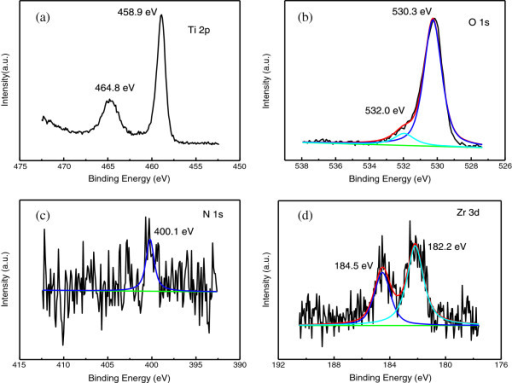
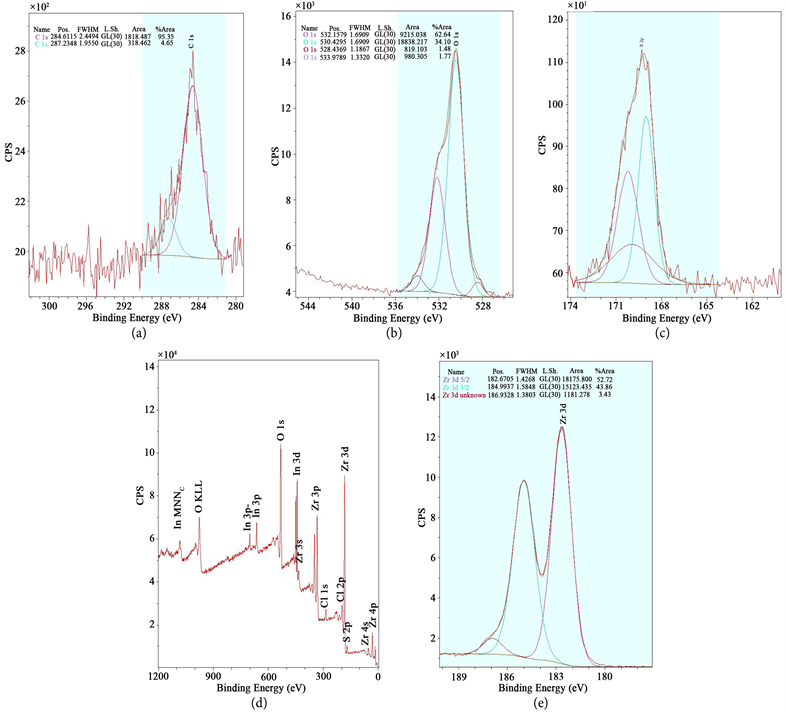
While in recent years, zirconia has been studied increasingly using surface science methods, the details of oxygen incorporation have not yet been tackled due to a lack of reliable model systems that mimic bulk properties reasonably well. 5 Especially when using (yttria-stabilized) zirconia as a solid-state electrolyte, its electronic structure and band alignment are crucial for its performance. 3 Furthermore, this process is of interest in catalysis, where zirconia is sometimes used as a catalyst, but mostly as support 4 oxygen spillover and reverse spillover would enable Mars–van-Krevelen-type reactions. 1 Introduction The spillover of oxygen from a metal or oxide onto zirconia (ZrO 2) is of immense importance in many applications, as ZrO 2 is used heavily as an oxygen-conducting electrolyte 1 in solid oxide fuel cells 2 and gas sensors. Our study also confirms that tetragonal ZrO 2 is stabilized via oxygen vacancies and shows that the XPS binding energy difference between O 1s and Zr 3d solely depends on the crystallographic phase. This can be either a metal deposited on the film, or, if the film has holes, the metallic (in our case, Rh) substrate. The vacancies can be filled via oxygen spillover from a catalyst that enables O 2 dissociation. These core level shifts have the opposite direction than what is usually encountered for reduced transition metal oxides. Due to the large band gap of zirconia (≈5–6 eV), these charges shift the electron levels, leading to higher binding energies of reduced tetragonal films w.r.t. This difference is explained by positively charged oxygen vacancies in the tetragonal films, which are slightly reduced. The results are also compared with density functional theory (DFT) calculations, which suggest that sites with strong H 2O adsorption contain twofold-coordinated oxygen.X-ray photoelectron spectroscopy (XPS) of five-monolayer-thick ZrO 2 films reveals a core level binding energy difference of up to 1.8 eV between the tetragonal and monoclinic phase. To validate our model system, transmission Fourier-transform infrared absorption spectroscopy (FTIR) studies at near-ambient pressures were carried out on monoclinic zirconia powder, showing comparable adsorption energies as TPD on the ultrathin film.
Nevertheless, the defect density is much smaller than in previous studies of H 2O/ZrO 2. Additional defect sites are created by multiple water adsorption/desorption cycles these water-induced changes were also probed by CO 2 TPD. STM shows that the defects with the strongest H 2O adsorption are found above subsurface dislocations. The monolayer TPD peak (180 K, desorption barrier 0.57 ± 0.04 eV) has a tail towards higher temperatures, caused by recombinative desorption from defect sites with dissociated water. The saturation coverage is one H 2O per surface Zr atom, with about 12% dissociation. We present a comprehensive study of water adsorption and desorption on an ultrathin trilayer zirconia film using temperature programmed desorption (TPD), X-ray photoelectron spectroscopy (XPS), as well as scanning tunneling microscopy (STM) at different temperatures.


 0 kommentar(er)
0 kommentar(er)
